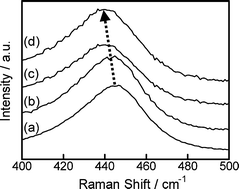
Nb-doped TiO2 particles were prepared by heating a mixture of peroxotitanic acid and peroxoniobic acid in air. When the heating temperature was more than 1173 K, the dominant phase obtained was rutile TiO2, along with a small amount of TiNb2O7. The relationship between the lattice parameters of the obtained rutile TiO2 depended on the molar fraction of Nb/(Ti + Nb). In the case where peroxo compounds were used as a precursor, a change in the lattice parameters of the rutile TiO2 was observed within the lower XNb range, as compared to the alkoxide method. The results indicate that a homogeneous dispersion of doped Nb5+ ions in the obtained rutile TiO2 lattice was achieved by using peroxo compounds. Furthermore, the oxide particles obtained by using peroxo compounds had a lower activation energy of the carrier electrons (Ea) and oxygen vacancies, even though the heating procedure was carried out in air. The UV-vis absorption spectra and Raman spectra of the obtained oxide particles indicated that the dominant reaction of the decomposition of O22− ions in the TiO2 lattice was O22− → O2 + 2e− as a reducing agent.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...