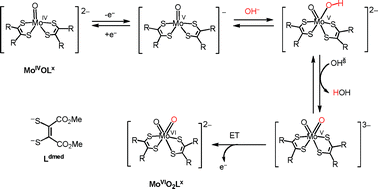
Electron transfer oxidation reaction of bis(dithiolene)monooxomolybdenum(IV) (MoIVOLx) complexes is studied as a model of oxidative-half reaction of arsenite oxidase molybdenum enzymes. The reactions are revealed to involve proton-coupled electron transfer. Electrochemical oxidation of MoIVOLx yields the corresponding bis(dithiolene)dioxomolybdenum(VI) complexes in basic solution, where the conversion of MoIVOLdmed supported by a smaller electron donating dithiolene ligand (1,2-dicarbomethoxyethylene-1,2-dithiolate, Ldmed) to MoVIO2Ldmed is faster than that of MoIVOLbdt with a larger electron donating dithiolene ligand (1,2-benzenedithiolate, Lbdt) under the same conditions. Titration experiments for the electrochemical oxidation reveal that the reaction involves two-electron oxidation and two equivalents of OH− consumption per MoIVOLx. In the conversion process of MoIVOLx to MoVIO2Lx, the five-coordinate bis(dithiolene)monooxomolybdenum(V) complex (MoVOLx) being a one-electron oxidized species of MoIVOLx is suggested to react with OH−. MoVOLx reacts with OH− in CH3CN or C2H5CN in a 2 : 2 ratio to give one equivalent MoIVOLx and one equivalent MoVIO2Lx, which is confirmed by the UV–vis and IR spectroscopies. The low temperature stopped-flow analysis allows investigations of the mechanism for the reaction of MoVOLx with OH−. The kinetic study for the reaction of MoVOLdmed with OH− suggests that MoVOLdmed reacts with OH− to give a six-coordinate oxo-hydroxo-molybdenum(V) species, MoVO(OH), and, then, the resulting species undergoes successive deprotonation by another OH− and oxidation by a remaining MoVOLdmed to yield the final products MoIVOLdmed and MoVIO2Ldmed complexes in a 1 : 1 ratio. In this case, the MoVO2 species are involved as an intermediate in the reaction. On the other hand, in the reaction of MoVOLbdt with OH−, coordination of OH− to the MoV centre to give a six-coordinate MoVO(OH)Lbdt species becomes the rate limiting step and other intermediates are not suggested. On the basis of these results, the ligand effects of the dithiolene ligands on the reactivity of the bis(dithiolene)molybdenum complexes are discussed.

 Please wait while we load your content...
Please wait while we load your content...