Surprising photochemical reactivity and visible light-driven energy transfer in heterodimetallic complexes†
Abstract
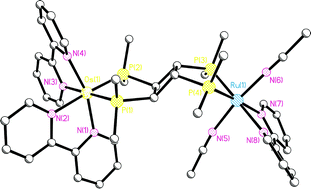
The bis(bidentate) phosphinecis,trans,cis-1,2,3,4-tetrakis(diphenylphosphino)cyclobutane (dppcb) has been used for the synthesis of a series of novel heterodimetallic complexes starting from [Ru(bpy)2(dppcb)]X2 (1; X = PF6, SbF6), so-called dyads, showing surprising photochemical reactivity. They consist of [Ru(bpy)2]2+ “antenna” sites absorbing light combined with reactive square-planar metal centres. Thus, irradiating [Ru(bpy)2(dppcb)MCl2]X2 (M = Pt, 2; Pd, 3; X = PF6, SbF6) dissolved in CH3CN with visible light, produces the unique heterodimetallic compounds [Ru(bpy)(CH3CN)2(dppcb)MCl2]X2 (M = Pt, 7; Pd, 8; X = PF6, SbF6). In an analogous reaction the separable diastereoisomers (ΔΛ/ΛΔ)- and (ΔΔ/ΛΛ)-[Ru(bpy)2(dppcb)Os(bpy)2](PF6)4 (5/6) lead to [Ru(bpy)(CH3CN)2(dppcb)Os(bpy)2](PF6)4 (9), where only the RuP2N4 moiety of 5/6 is photochemically reactive. By contrast, in the case of [Ru(bpy)2(dppcb)NiCl2]X2 (4; X = PF6, SbF6) no clean photoreaction is observed. Interestingly, this difference in photochemical behaviour is completely in line with the related photophysical parameters, where 2, 3, and 5/6, but not 4, show long-lived excited states at ambient temperature necessary for this type of photoreaction. Furthermore, the photochemical as well as the photophysical properties of 2–4 are also in accordance with their single crystal X-ray structures presented in this work. It seems likely that differences in “steric pressure” play a major role for these properties. The unique complexes 7–9 are also fully characterized by single-crystal X-ray structure analyses, clearly showing that the stretching vibration modes of the

 Please wait while we load your content...
Please wait while we load your content...