Electron delocalization and aromaticity in low-lying excited states of archetypal organic compounds†
Abstract
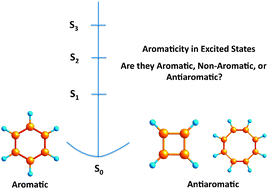
Aromaticity is a property usually linked to the ground state of stable molecules. Although it is well-known that certain excited states are unquestionably aromatic, the aromaticity of excited states remains rather unexplored. To move one step forward in the comprehension of aromaticity in excited states, in this work we analyze the electron delocalization and aromaticity of a series of low-lying excited states of
- This article is part of the themed collection: Aromaticity, electron delocalisation, and related molecular properties

 Please wait while we load your content...
Please wait while we load your content...