Hydrogen electrocatalysis on overlayers of rhodium over gold and palladium substrates—more active than platinum?
Abstract
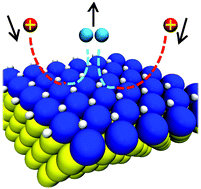
We have investigated the stability and catalytic activity of epitaxial overlayers of rhodium on Au(111) and Pd(111). Both surfaces show a strong affinity for hydrogen. We have calculated the energy of

 Please wait while we load your content...
Please wait while we load your content...