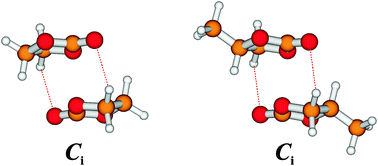
Dimers of cyclic carbonates: chirality recognition in battery solvents and energy storage†
Abstract
Dimers of ethylene carbonate and propylene carbonate are created in supersonic jet expansions and characterized by
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...