Jet-cooled hydrates of Chiral (S) 1,2,3,4-tetrahydro-3-isoquinoline methanol (THIQM): structure and mechanism of formation
Abstract
The mechanism of formation of
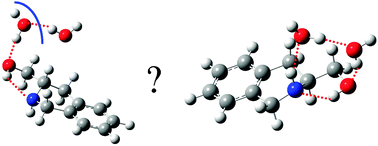
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...