Modeling van der Waals interactions between proteins and inorganic surfaces from time-dependent density functional theory calculations†
Abstract
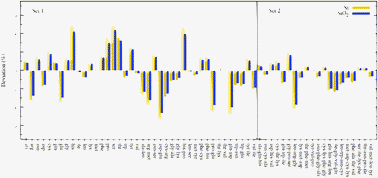
In this work we show how the ab initio determination of van der Waals coefficients within time-dependent density functional theory can be used to build efficient and accurate atomistic models that describe the long-range interactions of

 Please wait while we load your content...
Please wait while we load your content...