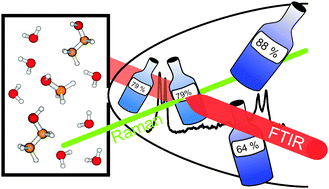
The vibrational dynamics of vacuum-isolated hydrogen-bonded complexes between water and the two simplest alcohols is characterized at low temperatures by Raman and FTIR spectroscopy. Conformational preferences during adaptive aggregation, relative donor/acceptor strengths, weak secondary hydrogen bonding, tunneling processes in acceptor lone pair switching, and thermodynamic anomalies are elucidated. The ground state tunneling splitting of the methanol–water dimer is predicted to be larger than 2.5 cm−1. Two types of alcohol–water trimers are identified from the spectra. It is shown that methanol and ethanol are better hydrogen bond donors than water, but even more so better hydrogen bond acceptors. As a consequence, hydrogen bond induced red shifts of OH modes behave non-linearly as a function of composition and the resulting cluster excess quantities correspond nicely to bulk excess enthalpies at room temperature. The effects of weak C–H⋯O hydrogen bonds are quantified in the case of mixed ethanol–water dimers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...