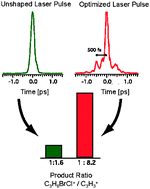
We report on the coherent control of the ultrafast ionization and fragmentation dynamics of the bromochloroalkanes C2H4BrCl and C3H6BrCl using shaped femtosecond laser pulses. In closed-loop control experiments on bromochloropropane (C3H6BrCl) the fragment ion yields of CH2Cl+, CH2Br+, and C3H3+ are optimized with respect to that of the parent cation C3H6BrCl+. The fragment ion yields are recorded in additional experiments in order to reveal the energetics of cation fragmentation, where laser-produced plasma radiation is used as a tunable pulsed nanosecond vacuum ultraviolet radiation source along with photoionization mass spectrometry. The time structure of the optimized femtosecond laser pulses leads to a depletion of the parent ion and an enhancement of the fragment ions, where a characteristic sequence of pulses is required. Specifically, an intense pump pulse is followed by a less intense probe pulse where the delay is 0.5 ps. Similarly optimized pulse shapes are obtained from closed-loop control experiments on bromochloroethane (C2H4BrCl), where the fragment ion yield of CH2Br+ is optimized with respect to that of C2H4BrCl+ as well as the fragment ion ratios C2H2+/CH2Br+ and C2H3+/C2H4Cl+. The assignment of the underlying control mechanism is derived from one-color 804 nm pump–probe experiments, where the yields of the parent cation and several fragments show broad dynamic resonances with a maximum at Δt = 0.5 ps. The experimental findings are rationalized in terms of dynamic ionic resonances leading to an enhanced dissociation of the parent cation and some primary fragment ions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...