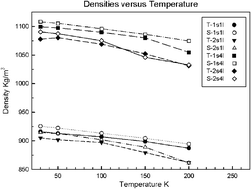
Classical equilibrium molecular dynamics (MD) simulations have been performed to investigate the dynamical and energetic properties in hydrogen and mixed hydrogen-tetrahydrofuran sII hydrates at 30 and 200 K and 0.05 kbar, and also at intermediate temperatures, using SPC/E and TIP4P-2005 water models. The potential model is found to have a large impact on overall density, with the TIP4P-2005 systems being on average 1% more dense than their SPC/E counterparts, due to the greater guest–host interaction energy. For the lightly-filled mixed H2-THF system, in which there is single H2 occupation of the small cage (1s1l), we find that the largest contribution to the interaction energy of both types of guest is the van der Waals component with the surrounding water molecules in the constituent cavities. For the more densely-filled mixed H2-THF system, in which there is double H2 occupation in the small cage (2s1l), we find that there is no dominant component (i.e., van der Waals or Coulombic) in the H2 interaction energy with the rest of the system, but for the THF molecules, the dominant contribution is again the van der Waals interaction with the surrounding cage-water molecules; again, the Coulombic component increases in importance with increasing temperature. The lightly-filled pure H2 hydrate (1s4l) system exhibits a similar pattern vis-à-vis the H2 interaction energy as for the lightly-filled mixed H2-THF system, and for the more densely-filled pure H2 system (2s4l), there is no dominant component of interaction energy, due to the multiple occupancy of the cavities. By consideration of Kubic harmonics, there is some evidence of preferential alignment of the THF molecules, particularly at 200 K; this was found to arise at higher temperatures due to transient hydrogen bonding of the oxygen atom in THF molecules with the surrounding cage-water molecules.

 Please wait while we load your content...
Please wait while we load your content...