Probing the interaction of amorphous solid water on a hydrophobic surface: dewetting and crystallization kinetics of ASW on carbon tetrachloride
Abstract
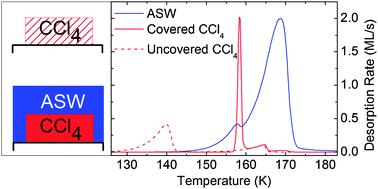
Desorption of carbon tetrachloride from beneath an amorphous solid
- This article is part of the themed collection: Physics and chemistry of ice and water

 Please wait while we load your content...
Please wait while we load your content...