Hemin interaction with bare and 4,4′-thio-bis-benzene-thiolate covered n-GaAs (110) electrodes
Abstract
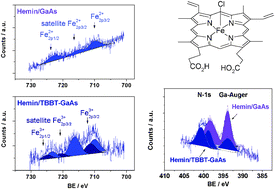
* Corresponding authors
a
Romanian Academy-Institute of Physical Chemistry “Ilie Murgulescu”, Spl. Independentei nr. 202, Bucharest, Romania
E-mail:
vlazarescu@icf.ro
Fax: 004 021 3121147
Tel: 004 0213167912
b National Institute of Material Physics, P.O. Box MG7, 77125 Bucharest, Romania
c Faculdad de Matemática, Astronomía y Física, Instituto de Física Enrique Gaviola (IFEG-CONICET), Universidad Nacional de Córdoba, 5000 Córdoba, Argentina
d Institute of Theoretical Chemistry, Ulm University, D-89069 Ulm, Germany
e University of Ulm, Albert-Einstein-Allee 11, D-89081 Ulm, Germany

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
L. Preda, C. Negrila, M. F. Lazarescu, M. Anastasescu, G. Dobrescu, E. Santos and V. Lazarescu, Phys. Chem. Chem. Phys., 2011, 13, 17104 DOI: 10.1039/C1CP21652J
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
