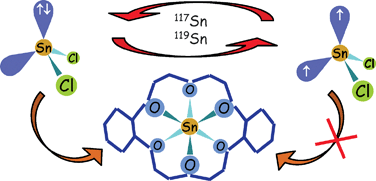
A novel approach is suggested to investigate the mechanisms of chemical complexation reactions based on the results of Fujii with co-workers; they have experimentally observed that several metals and metalloids demonstrate mass-independent isotope fractionation during the reactions with the DC18C6 crown ether using solvent-solvent extraction. In this manuscript, the isotope fractionation caused by the magnetic isotope effect is used to understand the mechanisms of chemical exchange reactions. Due to the rule that reactions are allowed for certain electron spin states, and forbidden for others, magnetic isotopes show chemical anomalies during these reactions. Mass-independent fractionation is suggested to take place due to the hyperfine interaction of the nuclear spin with the electron spin of the intermediate product. Moreover, the sign of the mass-independent fractionation is found to be dependent on the element and its species, which is also explained by the magnetic isotope effect. For example, highly negative mass-independent isotope fractionation of magnetic isotopes was observed for reactions of DC18C6 with SnCl2 species and with several Ru(III) chloro-species, and highly positive for reactions of this ether with TeCl62−, and with several Cd(II) and Pd(II) species. The atomic radius of an element is also a critical parameter for the reaction with crown ether, particularly the element ions with [Kr]4dn5sm electron shell fits the best with the DC18C6 crown ring. It is demonstrated that the magnetic isotope effect in combination with the theory of orbital hybridization can help to understand the mechanism of complexation reactions. The suggested approach is also applied to explain previously published mass-independent fractionation of Hg isotopes in other types of chemical exchange reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...