An intramolecular charge/electron transfer chemiluminescence mechanism of oxidophenyl-substituted 1,2-dioxetane
Abstract
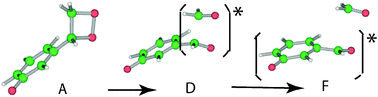
The chemiluminescence (CL) mechanism of oxidophenyl-substituted 1,2-dioxetane was investigated by performing TD-DFT calculations on biradicals of three model compounds. We propose a novel mechanism of CL in which excitation of a dissociative intermediate by infrared radiation (IRE) of the surrounding

 Please wait while we load your content...
Please wait while we load your content...