Understanding microsolvation of Li+: structural and energetical analyses†
Abstract
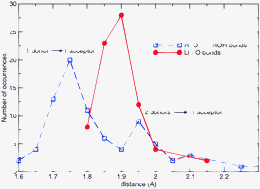
A stochastic exploration of the quantum conformational space for the (H2O)nLi+, n = 3, 4, 5 complexes produced 32 molecular clusters at the B3LYP/6–311++G** and MP2/6–311++G** levels. The first solvation shell is predicted to comprise a maximum of 4

 Please wait while we load your content...
Please wait while we load your content...