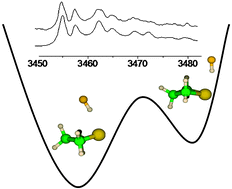
The coexistence of axial and equatorial hydrogen-bonded conformers of 1 : 1 (CH2)3S–HF (and –DF) has been observed in the same adiabatic expansion of a supersonic jet seeded with argon and in a static absorption cell at room temperature. High level calculations computed the axial conformer to be the most stable one with a small energy difference with respect to the equatorial one, in full agreement with previous microwave experiments. On the grounds of band contour simulations of FTIR spectra and ab initio energetic and anharmonic vibrational calculations, two pairs of νsHF donor stretching bands, observed in a series of jet-FTIR spectra at 3457.9 and 3480.5 cm−1 have been respectively assigned to the axial and equatorial forms of the 1 : 1 complex. In the jet-FTIR spectra series with HF, the assignment of an additional broad band (about 200 cm−1 higher in frequency with respect to νs) to a 1 : 2 complex has been supported by theoretical investigations. Experimental detection of both axial and equatorial forms of a cyclic trimer has been confirmed by calculated energetic and vibrational properties. The nature of hydrogen bonding has been examined within topological frameworks. The energetic partitioning within the 1 : 1 dimers has been elucidated with SAPT techniques. Interestingly, the interconversion pathway between two 1 : 1 structures has been explored and it was seen that the formation of the 1 : 1 complex affects the interconversion barrier on the ring puckering motion. The band contour analysis of gas phase FTIR experiments provided a consistent set of vibrational frequencies and anharmonic coupling constants, in good agreement with ab initio anharmonic vibrational calculations. Finally, from a series of cell-FTIR spectra recorded at different partial pressures of (CH2)3S and HF monomers, the absorption signal of the 1 : 1 complex could be isolated which enabled to estimate the equilibrium constant Kp = 0.023 at 298 K for the dimerization.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...