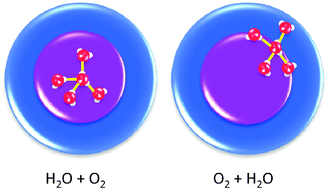
Core–shell particles with water clusters as the core and surrounded by an atomic or molecular shell have been synthesized for the first time by adding water and a co-dopant sequentially to helium nanodroplets. The co-dopants chosen for investigation were Ar, O2, N2, CO, CO2, NO and C6D6. These co-dopants have been used to investigate the effect of an outer shell on the ionization of the core material by charge transfer in helium nanodroplets. The specific aim was to determine how the identity of the shell material affects the fragmentation of water cluster ions, i.e. whether it helps to stabilize parent ion ((H2O)n+) formation or increases fragmentation (to form (H2O)nH+). N2, O2, CO2 and C6D6 all show a marked softening effect, which is consistent with the formation of a protective shell around the water cluster core. For CO and NO co-dopants, the response is complicated by secondary reactions which actually favour water cluster ion fragmentation for some water cluster sizes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...