Prediction of gas solubilities in ionic liquids†
Abstract
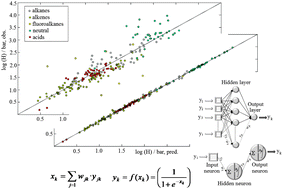
Ionic liquids (of which it is estimated that there are at least one million simple fluids) generate a rich chemical space, which is now just at the beginning of its systematic exploration. Many properties of ionic liquids are truly unique and, which is more important, can be finely tuned. Differential solubility of industrial chemicals in ionic liquids is particularly interesting, because it can be a basis for novel, efficient, environmentally friendly technologies. Given the vast number of potential ionic liquids, and the impossibility of a comprehensive empirical exploration, it is essential to extract the maximum information from extant data. We report here some computational models of gas solubility. These multiple regression- and neural network-based models cover a chemical space spanned by 48 ionic liquids and 23 industrially important gases. Molecular polarisabilities and special Lewis acidity and basicity descriptors calculated for the ionic liquid cations and anions, as well as for the gaseous solutes, are used as input parameters. The quality of fit “observed versus predicted Henry's law constants” is particularly good for the neural network model. Validation was established with an external dataset, again with a high quality fit. In contrast to many other neural network models published, our model is no “black box”, since contributions of the parameters and their nonlinearity characteristics are calculated and analysed.

 Please wait while we load your content...
Please wait while we load your content...