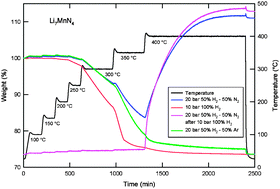
The hydrogen storage properties of Li7VN4 and Li7MnN4 were investigated both by experiment and by density functional theory calculations. Li7VN4 did not sorb hydrogen under our experimental conditions. Li7MnN4 was observed to sorb 7 hydrogen atoms through the formation of LiH, Mn4N, and ammonia gas. An applied pressurized mixture of H2/Ar and H2/N2 gases was helpful to mitigate the release of NH3 but could not prevent its formation. The introduction of N2 also caused weight gain of the sample by re-nitriding the absorbed products LiH and Mn4N, which correlated with the presence of Li2NH, LiNH2, and Mn2N detected by X-ray diffraction. While our observed results for Li7VN4 and Li7MnN4 differ in detail, they are in overall qualitative agreement with our theoretical work, which strongly suggests that both compounds are unlikely to form quaternary hydrides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...