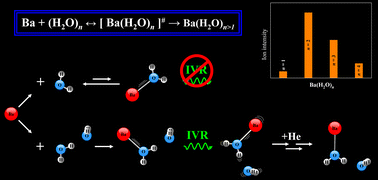
An experimental and theoretical study on the reactivity of neutral Ba atoms with water clusters has been conducted to unravel the origin of the irregular intensity pattern observed in one-photon ionization mass spectra of a Ba(H2O)n/BaOH(H2O)n−1 (n = 1–4) cluster distribution, which was generated in a laser vaporization-supersonic expansion source. The most remarkable irregular feature is the finding for n = 1 of a lower intensity for the Ba+(H2O)n peak with respect to that of BaOH+(H2O)n−1, which is opposite to the trend for n = 2–4. Rationalization of the data required consideration of a distinct behavior of ground-state and electronically excited state Ba atoms in inelastic and reactive Ba + (H2O)n encounters that can occur in the cluster source. Within this picture, the generation of Ba(H2O)n (n > 1) association products results from stabilizing collisions with atoms of the carrier gas, which are favored by intramolecular vibrational redistribution that operates on the corresponding collision intermediates prior to stabilization; the latter is unlikely to occur for Ba + (H2O) encounters. Overall, this interpretation is consistent with additional in-source laser excitation and quenching experiments, which aimed to explore qualitatively the effect of perturbing the Ba atom electronic state population distribution on the observed intensity pattern, as well as with the energetics of various possible reactions for the Ba + H2O system that derive from high level ab initio calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...