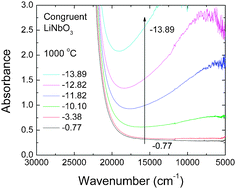
High-temperature optical in situspectroscopy was used to investigate the defect absorption, redox kinetics, and chemical diffusion of a lithium deficient (48.4 mol% Li2O) congruent melting lithium niobate single crystal (c-LN). Under reducing atmospheres of various oxygen activities, aO2, UV-Vis-NIR spectra measured at 1000 °C are dominated by an absorption band due to free small polarons centered at about 0.93 eV. The polaron band intensity was found to follow a power law of the form 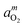 with m = −1/4. A chemical reduction model involving electrons localized on niobium ions on regular lattice sites can explain the observed defect absorption and its dependence on oxygen activity. The kinetics of reduction and oxidation processes upon oxygen activity jumps and the associated chemical diffusion coefficients are found in close agreement over a range from −0.70 to −14.70 in log aO2, indicating a reversible redox process. Assuming coupled fluxes of lithium vacancies and free small polarons for the attainment of stoichiometry, the diffusion coefficients of lithium vacancies as well as of lithium ions in the lithium deficient c-LN have been determined at 1000 °C.
with m = −1/4. A chemical reduction model involving electrons localized on niobium ions on regular lattice sites can explain the observed defect absorption and its dependence on oxygen activity. The kinetics of reduction and oxidation processes upon oxygen activity jumps and the associated chemical diffusion coefficients are found in close agreement over a range from −0.70 to −14.70 in log aO2, indicating a reversible redox process. Assuming coupled fluxes of lithium vacancies and free small polarons for the attainment of stoichiometry, the diffusion coefficients of lithium vacancies as well as of lithium ions in the lithium deficient c-LN have been determined at 1000 °C.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
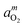 with m = −1/4. A chemical
with m = −1/4. A chemical 

 Please wait while we load your content...
Please wait while we load your content...