Proton coupled electron transfer of ubiquinone Q2 incorporated in a self-assembled monolayer†
Abstract
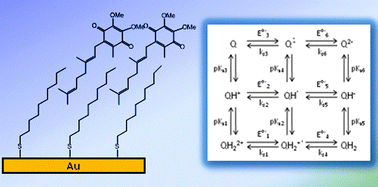
We present a complete study of the reduction of ubiquinone Q2 (UQ2) in simpler aqueous medium, over a pH range of 2.5 to 12.5. The short isoprenic chain ubiquinones (UQ2) were incorporated in a self-assembled monolayer. Under these conditions, the global 2e− electrochemical reaction can be described on the basis of a nine-member square scheme. The thermodynamic constants of the system were determined. The global 2e− process is controlled by the uptake of the second electron. The elementary electrochemical rate constants obtained by fitting of the experimental rate constant were ks4 = 1.5 s−1 for QH˙+2 ↔ QH2, ks5 = 1.5 s−1 for QH˙ ↔ QH− and ks6 = 1 s−1 for Q˙− ↔ Q2−. The three electrochemical reactions QH˙+2 ↔ QH2, QH˙ ↔ QH− and Q˙− ↔ Q2− are successively involved when increasing the pH. Protonations can occur or not, before or after the electron uptake and the reaction paths are, from low to high pH: e−, H+e−, e−H+, H+e−H+, H+e− and e−H+.

 Please wait while we load your content...
Please wait while we load your content...