Chemical evolution of biomolecule building blocks. Can thermodynamics explain the accumulation of glycine in the prebiotic ocean?†
Abstract
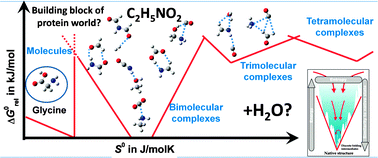
It has always been a question of considerable scientific interest why
* Corresponding authors
a
Department of Chemical Informatics, Faculty of Education, University of Szeged, Boldogasszony sgt. 6, Szeged 6725, Hungary
E-mail:
milan@jgypk.u-szeged.hu
Fax: +36 62 420 953
b Lehrstuhl für Theoretische Chemie, Institute für Physicalische und Theoretische Chemie, Universitat Bonn, Wegelerstraße 12, D-53115 Bonn, Germany
c Stereochemistry Research Group of the Hungarian Academy of Sciences, Dóm tér 8, H-6720 Szeged, Hungary
d Department of Chemistry, University of Toronto, Toronto, ON, Canada
It has always been a question of considerable scientific interest why

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. Szőri, B. Jójárt, R. Izsák, K. Szőri, I. G. Csizmadia and B. Viskolcz, Phys. Chem. Chem. Phys., 2011, 13, 7449 DOI: 10.1039/C0CP02687E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
