Determination of gas-phase ozonolysis rate coefficients of C8–14 terminal alkenes at elevated temperatures using the relative rate method†
Abstract
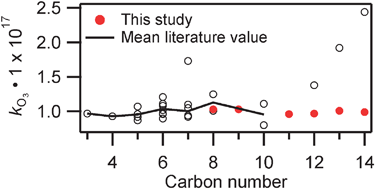
The rates of ozonolysis of a suite of terminal
- This article is part of the themed collection: Leuven gas kinetics meeting

 Please wait while we load your content...
Please wait while we load your content...