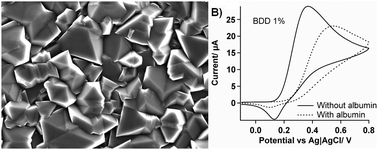
Fouling of electrode surfaces by electrode reaction products or by biological spectator species is known to inactivate electrochemical sensors and thus limit their use in biological conditions. Here we present an investigation on the stability of boron doped diamond (BDD) electrodes with different levels of doping. Three different doping levels were used (0.1, 1 and 5% in the carbon phase). The highly doped (5%) BDD is of particular interest as it is here used for the first time for biological applications. Three different redox reactions were examined based on their electrode reaction characteristics: ruthenium(III) hexaammine (outer sphere), ferrocyanide (suface dependent), dopamine (adsorption mediated). The effect of albumin at blood concentration was studied. All results were compared with glassy carbon.
There were no significant differences for the outer sphere electrochemistry, but all the BDDs showed improved resistance to fouling for the ferrocyanide oxidation. The electrocatalytic activity of BBD towards dopamine oxidation increased with increased boron content. However, this appears to be due to a larger number of defect sites which also increases the vulnerability to fouling by albumin and by electrode reaction products and the 5% BDD had similar properties to glassy carbon in this regard. These results suggest that it is possible to optimise the BDD performance for specific applications and that the large potential window for BDD may be due, at least in part, to its relatively poor electrocatalytic activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...