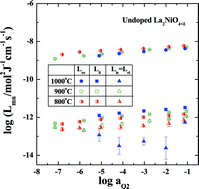
It is known [H.-S. Kim and H.-I. Yoo, Phys. Chem. Chem. Phys., DOI:10.1039/c0cp00722f] that all the isothermal mass and charge transport properties of a mixed ionic electronic conductor compound can be universally represented by a 2 × 2 Onsager transport coefficient matrix. Furthermore, the three independent coefficients of the matrix can be determined from a simple relaxation experiment under the ion-blocking condition in association with the equation of state with respect to the nonstoichiometry or thermodynamic factor. By using this method, we compile the transport matrices at 800 °C, 900 °C and 1000 °C, respectively, on the system of La2NiO4+δ across its entire stability range, and calculate thereby its transport properties to compare with the literature values available. The interference effect between mobile oxygen ions and holes upon their transfer is discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...