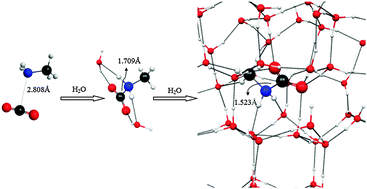
We investigated theoretically the interaction between methylamine (CH3NH2) and carbon dioxide (CO2) in the presence of water (H2O) molecules thus simulating the geometries of various methylamine–carbon dioxide complexes (CH3NH2/CO2) relevant to the chemical processing of icy grains in the interstellar medium (ISM). Two approaches were followed. In the amorphous water phase approach, structures of methylamine–carbon dioxide–water [CH3NH2/CO2/(H2O)n] clusters (n = 0–20) were studied using density functional theory (DFT). In the crystalline water approach, we simulated methylamine and carbon dioxide interactions on a fragment of the crystalline water ice surface in the presence of additional water molecules in the CH3NH2/CO2 environment using DFT and effective fragment potentials (EFP). Both the geometry optimization and vibrational frequency analysis results obtained from these two approaches suggested that the surrounding water molecules which form hydrogen bonds with the CH3NH2/CO2 complex draw the carbon dioxide closer to the methylamine. This enables, when two or more water molecules are present, an electron transfer from methylamine to carbon dioxide to form the methylcarbamic acid zwitterion, CH3NH2+CO2−, in which the carbon dioxide is bent. Our calculations show that the zwitterion is formed without involving any electronic excitation on the ground state surface; this structure is only stable in the presence of water, i.e. in a methyl amine–carbon dioxide–water ice. Notably, in the vibrational frequency calculations on the methylcarbamic acid zwitterion and two water molecules we find the carbon dioxide asymmetric stretch is drastically red shifted by 435 cm−1 to 1989 cm−1 and the carbon dioxide symmetric stretch becomes strongly infrared active. We discuss how the methylcarbamic acid zwitterion CH3NH2+CO2− might be experimentally and astronomically identified by its asymmetric CO2 stretching mode using infrared spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...