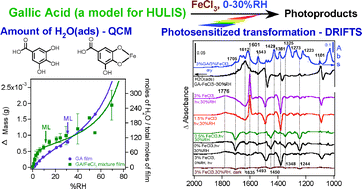
Little is known about the interfacial photochemistry of transition metal cations and chromophores relevant to atmospheric aerosols. We report herein water uptake and in situ surface-sensitive spectroscopic studies on the photosensitized transformation of solid gallic acid (GA), externally mixed with FeCl3 as a photosensitizer, under dry and humid conditions of <1% and 30% relative humidity (RH), respectively. GA is a hydrolysis product of tannic acid, a model macromolecule for humic-like substances (HULIS) in aerosols and aged polyaromatic hydrocarbons (PAH). Water uptake on GA and GA/FeCl3 mixture films was quantified using a quartz crystal microbalance (QCM) as a function of %RH (<1–60%). Results indicate continuous multilayer formation of adsorbed water with no phase transitions, with a monolayer of adsorbed water forming around 30 and 12%RH, respectively. Photochemical studies were conducted using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). Spectra were collected as a function of irradiation time (4 h), mass fraction of FeCl3 (0.5–3%) using irradiance of simulated solar light equivalent to 120 Wm−2 at 555 nm. Difference absorbance spectra show changes to GA functional groups suggesting the formation of organochlorine compounds in the condensed phase with their signature v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 1601 cm−1, and release of CO2. Potential halogenation pathways of GA in the presence of Fe3+ are discussed based on well-known aqueous phase chemistry. These pathways along with our results also suggest the release of volatile organochlorine compounds and Cl2 gas. Apparent first order rate constants, ks, of the photosensitized reactions were derived from kinetic curves of the most intense positive and negative spectral features at 1601 and 1381 cm−1, respectively. Values of ks at 120 Wm−2 are found to be higher than those reported from UV photo-Fenton reactions of GA in bulk aqueous phases containing H2O2, Fe2+ or Fe3+. The implication of our studies on the aging of multicomponent aerosols containing organic matter, transition metals and halide ions due to heterogeneous photochemistry is discussed.
C) at 1601 cm−1, and release of CO2. Potential halogenation pathways of GA in the presence of Fe3+ are discussed based on well-known aqueous phase chemistry. These pathways along with our results also suggest the release of volatile organochlorine compounds and Cl2 gas. Apparent first order rate constants, ks, of the photosensitized reactions were derived from kinetic curves of the most intense positive and negative spectral features at 1601 and 1381 cm−1, respectively. Values of ks at 120 Wm−2 are found to be higher than those reported from UV photo-Fenton reactions of GA in bulk aqueous phases containing H2O2, Fe2+ or Fe3+. The implication of our studies on the aging of multicomponent aerosols containing organic matter, transition metals and halide ions due to heterogeneous photochemistry is discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 1601 cm−1, and release of CO2. Potential
C) at 1601 cm−1, and release of CO2. Potential 

 Please wait while we load your content...
Please wait while we load your content...