Molecular mechanisms of the photostability of indigo†
Abstract
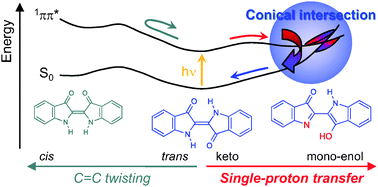
The photophysics of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the 1ππ* excited state. The nearly barrierless
O groups in the 1ππ* excited state. The nearly barrierless ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond has been investigated for bispyrroleindigo. It has been found that the twisting of the central C
C bond has been investigated for bispyrroleindigo. It has been found that the twisting of the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is unlikely to play a role in the photochemistry of
C bond is unlikely to play a role in the photochemistry of

 Please wait while we load your content...
Please wait while we load your content...