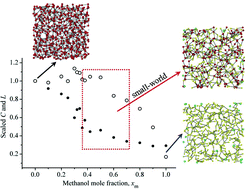
Statistical mechanics based topological analysis and island (or cluster) statistics were used to study the hydrogen bond (H-bond) networks in the water–methanol mixtures with the following methanol mole fractions (xm): 0.00, 0.10, 0.20, 0.25, 0.28, 0.30, 0.32, 0.36, 0.38, 0.42, 0.50, 0.60, 0.70, 0.80, 0.90, 1.00. NPT-Monte Carlo simulations were performed at room conditions using the TIP5P model potential for water and united-atoms (OPLS) for methanol to generate the H-bond networks. We have found evidence for non-ideal behavior of mixtures with xm ≈ 0.3. Several structural and topological properties present strong dependence with the mixture composition. Island statistics indicate a change from the percolated to non-percolate regime at xm ≈ 0.5. Statistical analysis of the islands' nature (homo-clusters: same type of molecules × hetero-clusters: two types of molecules) yields a preferential formation of homo-clusters that quantifies the local composition and preferential solvation (“microimmiscibility”). The topology of the hydrogen bond networks was characterized by local (clustering coefficients, average degrees), semi-global (path lengths) and global (spectral densities) properties. Small-world patterns (highly clustered and small path lengths) appear for xm in the range 0.40–0.70, and the momenta in the spectral densities correlate quite well with previous analysis based on rings, chains and branched chains topologies. It also seems that small quantities of methanol in water cause disruption of the continuous fully connected H-bond networks formed by water molecules.

 Please wait while we load your content...
Please wait while we load your content...