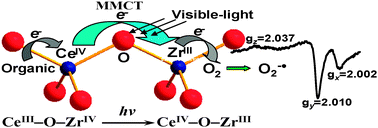
Tetragonal ZrO2–CeO2 solid solutions with composition Zr1−xCexO2 (x = 0.1, 0.2, and 0.3) were synthesized in a citrate complexation route and characterized by XRD, XPS, UV-vis diffuse reflectance and ESR measurements. The formation of the homogeneous solid solution Zr1−xCexO2 constructed the oxo-bridged bimetallic ZrIV−O−CeIII linkage between two neighboring flattened tetrahedrons of the structural framework. As compared to their parent oxides, the ZrO2–CeO2 solid solutions exhibited optical absorption extending to longer wavelengths in the visible region. The red shift in the absorption spectrum was demonstrated to be partially due to a metal-to-metal charge transfer (MMCT) transition of the oxo-bridged ZrIV−O−CeIII linkage. The visible-light induced MMCT transition of ZrIV−O−CeIII → ZrIII−O−CeIV resulted in the generation of the additional Ce(IV) and superoxide anion radical formed by the interaction of Zr(III) with adsorbed O2. Catalytic activity evaluation revealed that the photoexcitation of the MMCT over the solid solution can initiate the degradation of RhB and 2,4-DCP upon visible-light irradiation, whereby Zr(III) and Ce(IV) act as a site-specific reducing and oxidizing center, respectively. The structure of the solid solution Zr1−xCexO2 and the oxidation states of Zr and Ce species are also discussed in detail.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...