Chiral recognition between 1-(4-fluorophenyl)ethanol and 2-butanol: higher binding energy of homochiral complexes in the gas phase†
Abstract
Diastereomeric adducts between
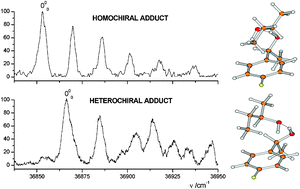
- This article is part of the themed collection: Homochirality and the origin of life

 Please wait while we load your content...
Please wait while we load your content...