Structure and dynamics of the Zr4+ ion in water
Abstract
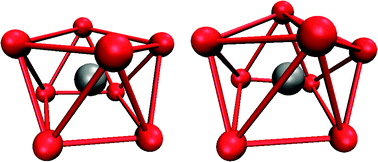
The four-times positively charged zirconium ion in aqueous solution was simulated, using an ab initio quantum mechanical charge field molecular dynamics approach. As no hydrolysis reaction occurred during the simulation time of 10 ps, the target of this study was the evaluation of the structure and dynamics of the monomeric hydrated zirconium(IV) ion. The ion forms three hydration shells. In the first hydration shell the ion is 8-fold coordinated with a maximum probability of the Zr–O distance at 2.25 Å. While no exchanges occurred between the first and second shell, the mean residence time of the

 Please wait while we load your content...
Please wait while we load your content...