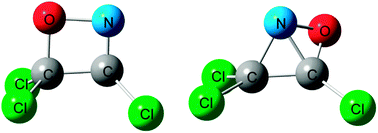
The products and mechanisms of the atmospherically and environmentally important reaction, C2Cl3 + NO, are investigated comprehensively by step-scan time-resolved Fourier transform infrared emission spectroscopy and the CCSD(T)/6-311+G(d)//B3LYP/6-311G(d) level of electronic structure calculations. Vibrationally excited products of Cl2CO, ClNCO, CCl3NCO and NCO have been observed in the IR emission spectra. Cyclic intermediates are found to play important roles leading to the rich variety of the chemical transformations of the reaction. Mainly two competitive reaction pathways are revealed: the four-membered ring intermediate pathway leading to the products Cl2CO + ClCN which is essentially barrierless and the bicyclic ring intermediate pathway leading to the product channels of ClNCO + CCl2,CCl3NCO and CCl3 + NCO which is rate-limited by a barrier of 42.9 kJ mol−1 higher than the reactants. By photolyzing the precursor at 248 and 193 nm, respectively, C2Cl3 radicals with different internal energy are produced to observe the product branching ratios as a function of reactant energy. The Cl2CO channelvia the four-membered ring intermediate pathway is shown to be overwhelmingly dominant at low energy (temperature) but become less important at high energy while the ClNCO and CCl3NCO channels via the bicyclic ring intermediate pathway are greatly enhanced and compete effectively. The experimental observation of the products and their branching ratios varying with reactant energy is well consistent with the calculated potential energy profiles.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...