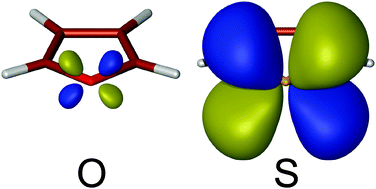
We have used a potential wall method to investigate the role of d orbitals in the a2 singly-occupied molecular orbitals of 2A2 negative ion states of two molecular series: pyridine, phosphabenzene, arsabenzene, stibabenzene (C5H5X, X = {N, P, As, Sb}), and furan, thiophene, selenophene, tellurophene (C4H4X, X = {O, S, Se, Te}). Unlike for the lower lying doubly occupied orbitals, heteroatom d–carbon p in-phase (bonding) interactions in these a2 orbitals are clearly identified and explain the 0.5 eV stabilization of the 2A2 radical anion state in those compounds where the heteroatoms have d orbitals in the valence shell, compared to compounds where d orbitals are missing in the valence shell of the heteroatoms. The performance of both the potential wall approach and the approximate expression of Tozer and De Proft for calculating negative electron affinities has been also investigated, through a comparison with results obtained using electron-transmission spectroscopy experiments.

 Please wait while we load your content...
Please wait while we load your content...