A multivalent hexapod having 24 stereogenic centers: chirality and conformational dynamics in homochiral and heterochiral systems
Abstract
The monolayer formation of a chiral oligo(p-phenylene
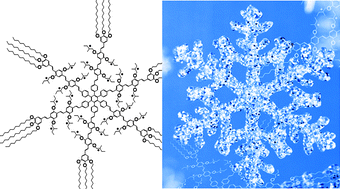
- This article is part of the themed collection: 2D Crystal Engineering

 Please wait while we load your content...
Please wait while we load your content...