Polymorphism of 5-(pyridin-2-ylmethylene)-3-phenyl-2-methylthio-3,5-dihydro-4H-imidazole-4-one†
Abstract
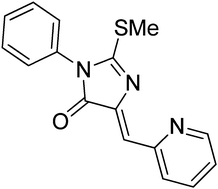
The title compound, C16H13N3OS (1), exists in three polymorphic forms. Crystalline 1 undergoes an enantiotropic, first-order, k2 phase transition at 262.9(5) K with ΔH = 0.3(1) kJ mol−1. Upon cooling below the transition temperature, the high temperature orthorhombic polymorph (Form I, space groupPbcm) transforms into a low temperature orthorhombic polymorph (Form II, space groupPbca) with a unit cell twice the size of that of the Form I. A molten 1 can be cooled in a controlled fashion to generate a monoclinic Form III of 1 with the unit cell size similar to that of Form I. Metastable Form III, once isolated, is indefinitely stable between 100 K and its melting point of 466 K. If crystals of Form III are in contact with seed crystals of Form I, a monotropic t2 first-order Form III → Form I phase transition occurs upon heating with the onset between 420 and 448 K and ΔH = −1.7(4) kJ mol−1. The most substantial differences among the molecular geometries of 1 in Forms I–III are observed in the position and tilt of the

 Please wait while we load your content...
Please wait while we load your content...