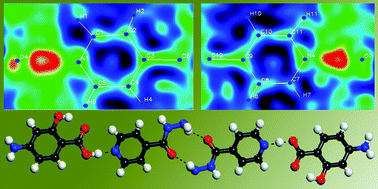
Drug-drug co-crystals: Temperature-dependent proton mobility in the molecular complex of isoniazid with 4-aminosalicylic acid†
Abstract
Two
- This article is part of the themed collection: Dynamic behaviour and reactivity in crystalline solids

 Please wait while we load your content...
Please wait while we load your content...