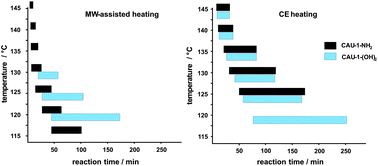
Two isoreticular aluminium containing metal–organic frameworks, CAU-1-NH2 and CAU-1-(OH)2, have been synthesized solvothermally using 2-aminoterephthalic and 2,5-dihydroxyterpththalic acid to elucidate the product formation depending on the functionalized carboxylic acids. Here, we focus on the product formation kinetics of the amino-functionalized CAU-1, i.e.CAU-1-NH2. The reactions were studied by in situ energy dispersive X-ray diffraction (EDXRD) experiments in the temperature range of 115–145 °C. Both conventional electric (CE) and microwave (MW) -assisted heating were employed. The latter led to shorter induction periods as well as shorter crystallisation times compared to the conventional synthesis. In analogy with the previously reported results of the EDXRD studies of the formation of CAU-1-(OH)2, similar crystal growth rates were observed for both CAU-1-NH2 and CAU-1-(OH)2 using CE heating. In contrast, MW-assisted synthesis of CAU-1-NH2 led to a shorter induction period as well as an acceleration of the crystal growth compared to CAU-1-(OH)2. The acceleration in the crystal growth stage of CAU-1-NH2 can be attributed to a larger pre-exponential factor (about three times that of CAU-1-(OH)2) derived from the Arrhenius plot. However, the activation energy of the crystal growth exhibits similar values for both MW-assisted and CE synthesis of CAU-1-NH2 and CAU-1-(OH)2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...