Competition between π–π or furan–perfluorophenyl stacking interactions in conjugated compounds prepared from azomethine connections†
Abstract
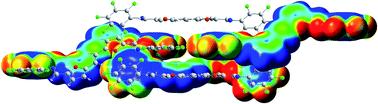
Conjugated compounds associating ![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) F contacts, π–π interactions and
F contacts, π–π interactions and ![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) F contacts and π–π interactions while the compound based on a furylene–vinylene unit shows a packing pattern exclusively due to strong
F contacts and π–π interactions while the compound based on a furylene–vinylene unit shows a packing pattern exclusively due to strong

 Please wait while we load your content...
Please wait while we load your content...