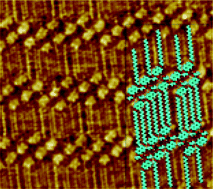
Enantiomers of a 2,6-napthyl derivative—bearing a long alkyl chain at one position and a lactate group at the other—were synthesised and the formation of self-assembled physisorbed monolayers of the molecules on highly oriented pyrolytic graphite was studied by scanning tunnelling microscopy. The results of imaging this monolayer reveal the rare formation of an irregular packing which has been analysed in detail. The enantiomers appear as chiral dimers on the surface. The alkyl chains direct the assembly to a large degree, since they run along the graphite axes, but the chiral lactate group coupled with the symmetry restrictions of the naphthyl ring apparently causes a frustration in the packing. The dimers pack into rows but their relative orientation along the long row axis is not fixed, inducing a degree of disorder in this direction. However, the molecular dimers are perfectly ordered in a direction nearly perpendicular to it, in an effective one-dimensional crystal. In contrast the racemic compound shows a less densely packed and defective monolayer, where relatively few nanoscopic domains of apparently enantiopure compound are interspersed in the disordered array. These results shine light on the self-assembly mechanism and are relevant for understanding packing in three dimensions, and especially for chiral compounds which do not form ordered crystals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...