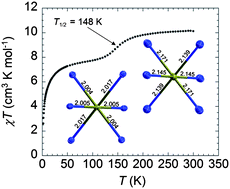
The synthesis, structures and magnetic properties of a new trinuclear spin crossover complex, [FeII3(npt)6(EtOH)4(H2O)2](ptol)6·4EtOH (1), and of its CoII (2 and 3) and NiII (4) analogues, are reported here. The complexes were synthesized by reacting a 1,2,4-triazole-based ligand, 4-(4′-nitrophenyl)-1,2,4-triazole (npt), with the p-tolylsulfonate (ptol) metal salts in methanol or ethanol. Structural analyses revealed that all complexes are iomorphous and consist of a linear trinuclear core where metal centres are bridged by triazole groups. For 1, dc susceptibility measurements exhibit gradual spin transition with T1/2 = 148 K which corresponds to HS → LS crossover for the triazole bridged central FeII ion. This spin transition was confirmed by Single-Crystal X-Ray Diffraction data of 1 at 100 K and 181 K, where the low temperature measurement revealed a decrease in volume for the central FeII ion, which is in agreement with a HS → LS transition.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...