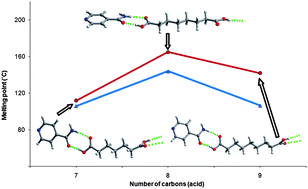
The crystal structures and melting point properties of isonicotinamide cocrystals with alkanediacids HO2C(CH2)n−2CO2Hn = 7–9†
Abstract
The tunability of physiochemical properties of crystalline materials through controlled cocrystal formulation is an aim for both the

 Please wait while we load your content...
Please wait while we load your content...