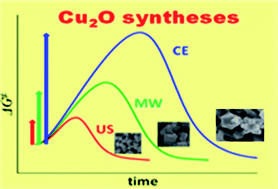
Ultrasound (US) and microwave (MW) irradiation has been applied for rapid syntheses of cuprous oxides from copper sulfate, sodium citrate, sodium carbonate and polyvinylpyrrolidone in water at a relatively low temperature below 50 °C. Compared with conventional electric (CE) synthesis, US and MW heating lead very rapidly to cuprous oxides having a well-defined small cubic morphology in high yield. The rapid syntheses with US (acceleration degree: 48–81 times of CE synthesis) and MW (acceleration degree: 17–18 times of CE synthesis) are due to decreased activation free energy (ΔG≠, of the Eyring equation) or increased pre-exponential factor (A, of the Arrhenius equation). However, the activation energy (Ea) decreases in the order of US > MW > CE synthesis. The decreased activation free energy is due to a small decrease (or relatively high) in activation entropy (ΔS≠) rather than a decreased activation enthalpy (ΔH≠). The accelerated syntheses do not depend noticeably on reaction stages (nucleation and crystal growth) and synthesis methods (US and MW, excluding the acceleration degrees), suggesting the acceleration is mainly due to physical effects including hot spots or transient temperature rather than chemical ones.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...