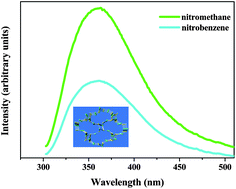
Hydrothermal reactions of ZnII or CuII ion with 1-hydroxyl-2-(3-pyridyl)ethylidene-1,1-diphosphonic acid (H4L) afforded two metal phosphonates, namely, [Zn2(L)(H2O)] (1) and [Cu4(L)2 (H2O)4]·(H2O)1.25 (2). In 1, [ZnO3N], [ZnO4] and [PO3C] tetrahedra are connected through corner-sharings to form a 3D framework with a 14MR ellipsoid-like channel. In 2, the [PO3C] tetrahedron also shares corners with a copper square-pyramid ([CuO4N], [CuO5]) into a hybrid layer, which is further pillared by L4− anion into a 3D neutral framework. Solid 1 is thermally stable up to 250 °C under an air atmosphere and displays UV luminescence. More interestingly, the dehydrated phase of 1 is selective and reversible for sensing of nitrobenzene. And the magnetic property of 2 has also been studied.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...