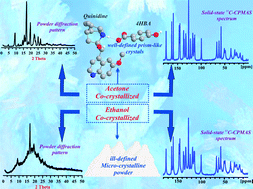
Based on crystal engineering principles, we have explored the predictability of resulting structures of a multi-component pharmaceutical model complex derived from 4-hydroxybenzoic acid (4HBA) and quinidine, an anti-malarial constituent of Cinchona tree bark. Though the obtained complex is stabilized by a slightly different set of charge-assisted heterosynthons as proposed, the applied concept was efficient in predicting the salt formation. The salt 1 crystallizes in a monoclinic space group [P21 (no. 4), Z = 8, a = 6.914 Å, b = 36.197 Å, c = 9.476 Å and β = 92.126], where the asymmetric unit is comprised of two quinidine and two 4HBA molecules. In addition, a micro-crystalline, less-defined sample of 1 was obtained from rapid co-crystallization in ethanol and successfully identified via both infrared spectroscopy and multinuclear solid-state NMR. The interpretation of the obtained NMR data was supported by DFT quantum-chemical computations while illustrating options of “NMR crystallography”.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...