Stereospecific solid-state cyclodimerization of bis(trans-2-butenoato)calcium and triaquabis(trans-2-butenoato)magnesium†‡
Abstract
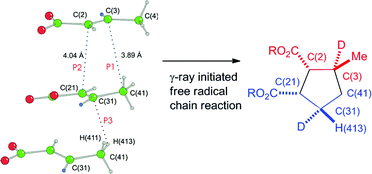
60Co γ-irradiation of bis(trans-2-butenoato)calcium (4) and triaquabis(trans-2-butenoato)magnesium (11) affords cyclodimerization products cis,trans- and trans,trans-nepetic acids (5 and 15), respectively. The products are produced in high yield, based upon conversion, by a γ-ray induced radical chain pathway and provide unprecedented, stereospecific, one-pot syntheses of two of the four possible nepetic acid diastereomers, adding significant generality to the synthetic scope of the γ-ray induced reactions of crystalline metal trans-2-butenoates. Stereochemical analysis of the products from the analogous trans-2-butenoato-3-d complexes 19 and 27 establishes unequivocally that hydrogen atom transfer is also stereospecific and not part of a random process. The stereochemistry of the diastereomers is that predicted by the analysis of crystal packing, consistent with the least-motion principles of the topochemical postulate. Analysis of the crystal structures, with respect to the nearest neighbors, is consistent with the hypothesis that the formation of both carbon–carbon bonds and hydrogen atom transfer are topochemical and controlled by the crystal lattice. Analysis of the packing diagrams provides a pathway for chain propagation throughout the crystal that consumes all the molecules in the unit cell.
- This article is part of the themed collection: Reactions in molecular solids and host–guest systems

 Please wait while we load your content...
Please wait while we load your content...