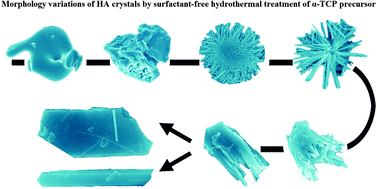
In the present work, an unusual precursor, α-tricalcium phosphate [Ca3(PO4)2, α-TCP] was used for the modulation of hydroxyapatite [Ca10(PO4)6(OH)2, HA] crystals. Micro-structure and morphology controllable HA crystals were successfully synthesized via direct hydrothermal treatment of α-TCP particles in the absence of any surfactants or additives, and were investigated by field emission scanning electron microscopy, field emission transmission electron microscopy, X-ray diffraction, X-ray fluorescence spectroscopy and Fourier transform infrared spectroscopy. Well developed HA crystals with different structures and morphologies (chrysanthemum-like HA microflowers, enamel-like HA microparticles, rectangle shaped HA microplates and HA microrods) could be obtained by adjusting the reaction temperature and the concentration of Ca2+ ions. The experimental results showed that different aggregation routes of HA nanorods which grow along the c-axis were the reason for the formation of various micro-structures and morphologies. The possible mechanism of the formation of HA crystal was based on the Cluster Growth Model. This study suggested that the hydrothermal phase transformation from α-TCP to HA could be a promising way for the delicate morphology control of HA crystals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...