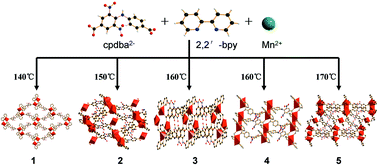
A series of Mn-cpdba-2,2′-bpy complexes, {[Mn(cpdba)(2,2′-bpy)]}n1, {[Mn2(cpdba)2(2,2′-bpy)(H2O)2]·3H2O}n2, {[Mn2(cpdba)2(2,2′-bpy)2(H2O)2]·H2O}n3, {[Mn(cpdba)(2,2′-bpy)]·1.5H2O}n4, {[Mn4(cpdba)4(2,2′-bpy)3(H2O)2]}n5 (H2cpdba = 4-(4-carboxyphenylamino)-3,5-dinitrobenzoic acid; 2,2′-bpy = 2,2′-bipyridine) have been synthesized at different temperatures under hydrothermal conditions. Complex 1 has a 2D (4,4) network with alternate left and right-handed helical chains. With increasing temperature to 150 °C, complex 2, which has a complicated 3D framework constructed by a unique building block Mn3(cpdba)6(2,2′-bpy)2, can be obtained. Complex 3 that has been reported previously was obtained at 160 °C. This complex exhibits a 1D chain structure which is extended into a 3D supermolecular framework by π–π stacking interactions. Complex 4 was detected as a by-product in the synthesis of 3. X-Ray single crystal diffraction analysis reveals the formation of 2D layer network containing [Mn(CO2) + Mn(CO2)]n double-helices. With continuing increase to 170 °C, complex 5 featuring 3D framework similar to 2, with the decrease in the coordinated water and lattice water molecules as well as increase in the regularity of the framework, was formed. Influence of the 2,2′-bpy co-ligand on the coordination configuration of the cpdba2− ligand has been discussed. The magnetic susceptibility measurements of 1 and 5 reveal that two complexes exhibit antiferromagnetic coupling interactions.

 Please wait while we load your content...
Please wait while we load your content...